Yesterday I was listening to a news segment on CBC Links to an external site.about cervical cancer screening results that currently have 6-month delays in parts of Canada due to post-pandemic backup and staff shortages. Coincidently, while researching beta-galactosidase, I discovered that this enzyme is an important biomarker for certain cancers, including ovarian cancer (Fan et al., 2021) and, most recently, has been found to be over-expressed in gastric cancer as well (Kubo et al., 2021).
Human beta-galactosidase is highly expressed in senescent cells, which are cells that stop dividing but continue to exist and metabolize. Accumulation of senescent cells is an indicator of aging in general, and the presence of these cells is associated with a risk for many diseases including cancer, osteoarthritis, atherosclerosis (arterial plaque build-up), Alzheimer’s, diabetes, and more (Childs et al., 2017) (Chen et al., 2022). Interestingly, cellular senescence seems to be one of the body’s defenses against tumor growth, in that it’s a way for the body to stop the division of cells that have shortened telomeres and may have mutations (Campisi, 2001). Yet, secretions by senescent cells also cause disease (Childs et al., 2017) and even a small number of senescent cell secretions such as cytokines can have a significant impact on cells throughout the entire body and cause or increase disease (Burton, 2009). And so, the presence of an accumulation of human beta-galactosidase in tissues indicates aging and the onset or development of disease.
Early cancer detection greatly affects survival rates. Moreover, distinguishing the exact type of cancer aids in treatment decisions. Finding biomarkers that indicate early cancer growth and specify the type of cancer is key to increasing survivorship. Beta-galactosidase is shown to be present in ovarian cancer and most recently associated with gastric cancer (Kubo et al., 2021). Detection of beta-galactosidase has been historically done by a technology called X-gal but has certain disadvantages including that it can only be done in cells that are non-living and the staining takes a long time and is difficult to visualize. However, a new technology called Cellular Senescence Detection Kit - SPiDER-βGal is more sensitive, easier to see, and can be done on live cells (in vivo) (Cellular Senescence Detection Kit - SPiDER-βGal, n.d.).
The image shows beta-galactosidase cells illuminated with the application of SPiDER-βGal technology. Source: (Cellular Senescence Detection Kit - SPiDER-βGal, n.d.).
Research has shown that beta-galactosidase fluorescent illumination is a reliable indicator for gastric cancer, liver, and other cancers. B-galactosidase is found in higher concentrations in tumor tissues compared to healthy cells. The presence of beta-galactosidase can be seen visually, with a stronger fluorescence indicating higher concentrations. Furthermore, the detection is fast and can be seen only 10 minutes after application. This technology could thus be used during surgery, to help surgeons see cancer cells during surgery, namely fluorescence-guided surgery (Ogawa et al., 2021). Also, it can shine for hours, is non-toxic, and will illuminate even at low concentrations. Detecting and removing even small amounts of cancer cells can greatly decrease reoccurrences or metastasis.
I became curious to learn exactly how the probe lights up beta-galactosidase and it took hours of research to find some semblance of an explanation. I finally came upon J. Zhang et al., the researchers who originally developed the technology and published their findings, in 2017. The probe is a substrate for Beta-galactosidase and ‘turns on’ in a solution with H2O. It can detect even a small concentration of 0.1 nM and has a very high bonding affinity. The probe can distinguish between endogenous Beta-galactosidase cells and non-endogenous. It has a hemicyanine structure (meaning dyes with two aromatic groups) with a D-galactose residue using a glycosidic bond and s fluorescent intensity at 703nm (J. Zhang et al., 2017).
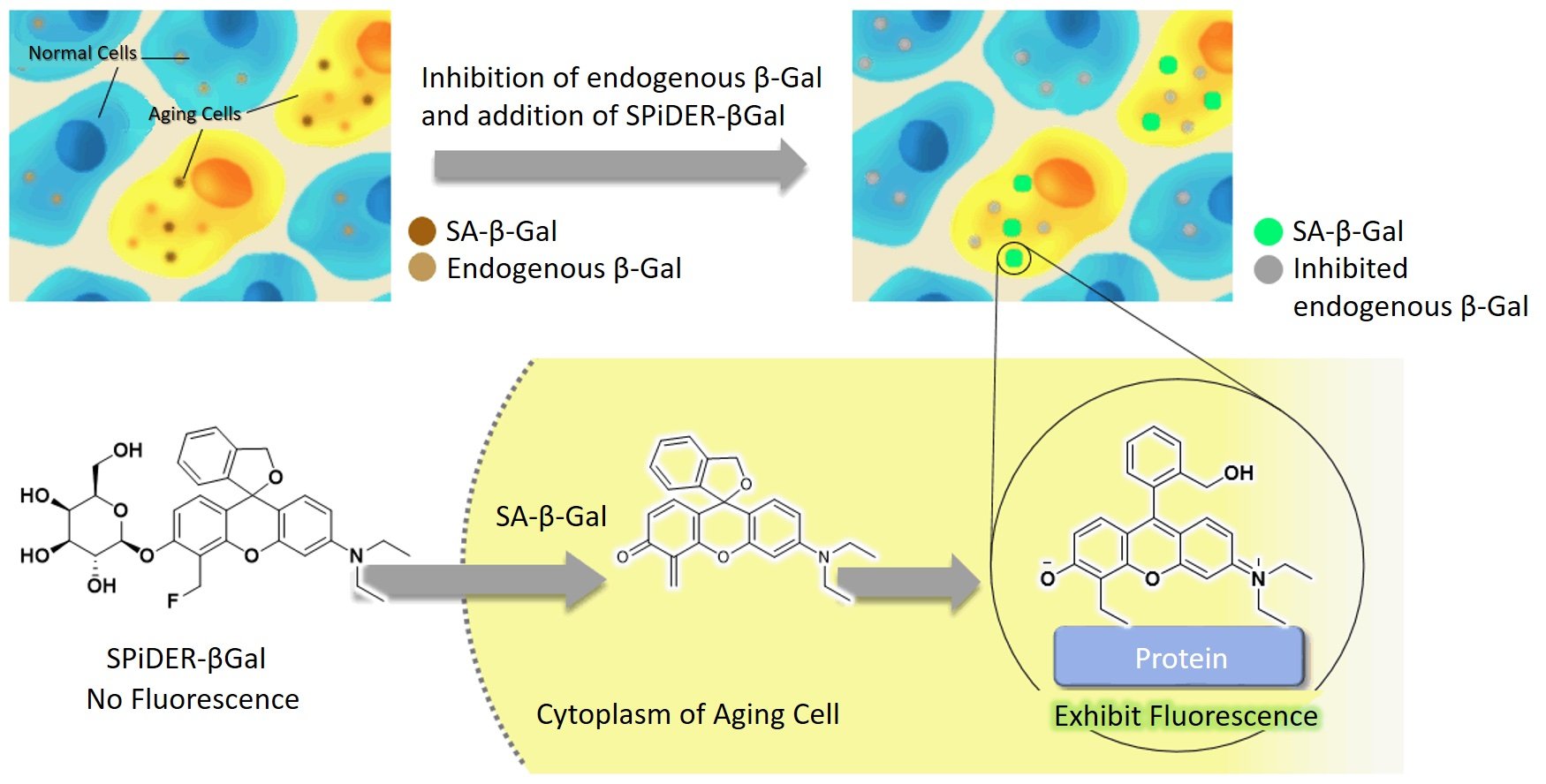
I will admit I am still not satisfied with this information and want to really understand how this probe lights up the specific substrate groups. Here is an image that I partially understand. I can see that Gal-Pro is the probe. It meets with Beta-galactosidase. The top sugar (chair-shaped) comes off and binds to beta-galactosidase. I have many remaining questions, such as why it bonds and how it illuminates. I am sure I am limited in my understanding as my organic chemistry knowledge is not comprehensive. Perhaps someone can comment and explain what happens on a substrate level and what causes the fluorescence.
Image from Qiu et al., 2020.
In summary, beta-galactosidase is present in high concentrations in senescent cells and is an indicator of ovarian and gastric cancer. SPiDER-βGal probe is a powerful new technology that can be used for fluorescence-guided surgery to find and remove cancer cells. It can also be used as a fast biomarker in vivo for the presence of gastric and ovarian cancer, even in small concentrations.
REFERENCES
Kubo, H., Murayama, Y., Ogawa, S., Matsumoto, T., Yubakami, M., Ohashi, T., Kubota, T., Okamoto, K., Kamiya, M., Urano, Y., & Otsuji, E. (2021). β-Galactosidase is a target enzyme for detecting peritoneal metastasis of gastric cancer. Scientific Reports, 11(1), 10664. https://doi.org/10.1038/s41598-021-88982-2Links to an external site.
Fan, F., Zhang, L., Zhou, X., Mu, F., & Shi, G. (2021). A sensitive fluorescent probe for β-galactosidase activity detection and application in ovarian tumor imaging. Journal of Materials Chemistry. B, Materials for Biology and Medicine, 9(1), 170–175. https://doi.org/10.1039/d0tb02269aLinks to an external site.
Childs, B. G., Gluscevic, M., Baker, D. J., Laberge, R.-M., Marquess, D., Dananberg, J., & van Deursen, J. M. (2017). Senescent cells: an merging target for diseases of ageing. Nature Reviews. Drug Discovery, 16(10), 718–735. https://doi.org/10.1038/nrd.2017.116Links to an external site.
Chen, Y.-H., Zhang, X., Ko, K.-Y., Hsueh, M.-F., & Kraus, V. B. (2022). CBX4 regulates replicative senescence of WI-38 fibroblasts. Oxidative Medicine and Cellular Longevity, 2022, 5503575. https://doi.org/10.1155/2022/5503575
Childs, B. G., Gluscevic, M., Baker, D. J., Laberge, R.-M., Marquess, D., Dananberg, J., & van Deursen, J. M. (2017). Senescent cells: an emerging target for diseases of ageing. Nature Reviews. Drug Discovery, 16(10), 718–735. https://doi.org/10.1038/nrd.2017.116Links to an external site.
Campisi, J. (2001). Cellular senescence as a tumor-suppressor mechanism. Trends in Cell Biology, 11(11), S27-31. https://doi.org/10.1016/s0962-8924(01)02151-1
Burton, D. G. A. (2009). Cellular senescence, ageing and disease. Age (Dordrecht, Netherlands), 31(1), 1–9. https://doi.org/10.1007/s11357-008-9075-y
Cellular Senescence Detection Kit - SPiDER-βGal. (n.d.). Dojindo.eu.com. Retrieved December 8, 2022, from https://www.dojindo.eu.com/store/p/895-Cellular-Senescence-Detection-Kit-SPiDER-Gal.aspx
Ogawa, S., Kubo, H., Murayama, Y., Kubota, T., Yubakami, M., Matsumoto, T., Yamamoto, Y., Morimura, R., Ikoma, H., Okamoto, K., Kamiya, M., Urano, Y., & Otsuji, E. (2021). Rapid fluorescence imaging of human hepatocellular carcinoma using the β-galactosidase-activatable fluorescence probe SPiDER-βGal. Scientific Reports, 11(1), 17946. https://doi.org/10.1038/s41598-021-97073-1
Zhang, J., Li, C., Dutta, C., Fang, M., Zhang, S., Tiwari, A., Werner, T., Luo, F.-T., & Liu, H. (2017). A novel near-infrared fluorescent probe for sensitive detection of β-galactosidase in living cells. Analytica Chimica Acta, 968, 97–104. https://doi.org/10.1016/j.aca.2017.02.039
Qiu, W., Li, X., Shi, D., Li, X., Gao, Y., Li, J., Mao, F., Guo, Y., & Li, J. (2020). A rapid-response near-infrared fluorescent probe with a large Stokes shift for senescence-associated β-galactosidase activity detection and imaging of senescent cells. Dyes and Pigments: An International Journal, 182(108657), 108657. https://doi.org/10.1016/j.dyepig.2020.108657